Fascination About Philips CPAC Lawsuit
Table of ContentsThe 9-Second Trick For Philips CPAC LawsuitPhilips CPAC Lawsuit for DummiesThe smart Trick of Philips CPAC Lawsuit That Nobody is Talking AboutLittle Known Questions About Philips CPAC Lawsuit.
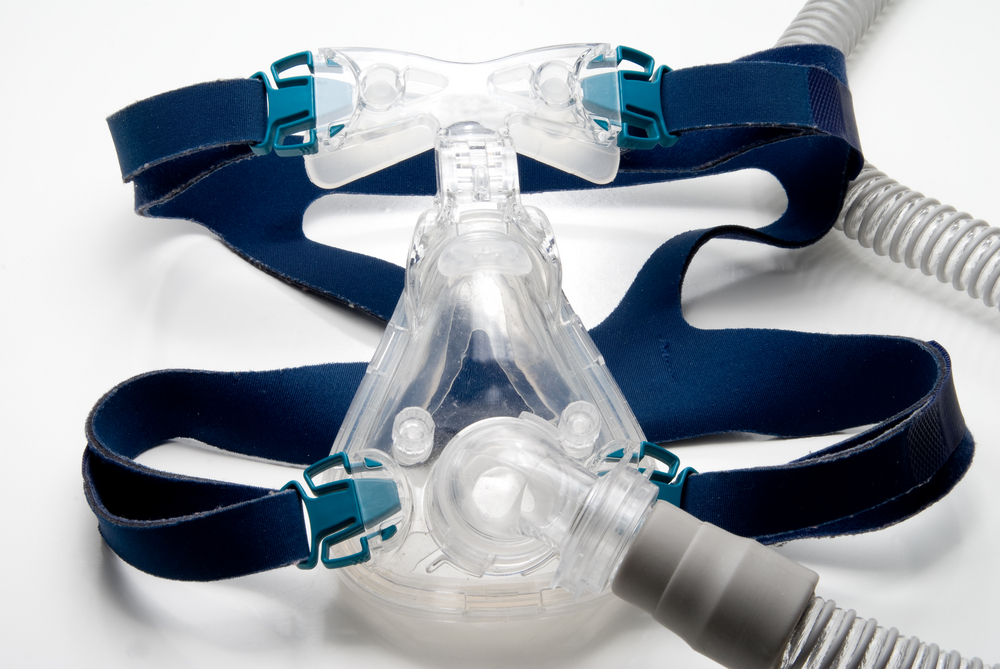
For instance, keeping CPAP makers in places can also increase the destruction rate. This may be challenging to control in some places. PE-PUR audio reduction foam is a polyester-based polyurethane foam that is not hazardous when it is undamaged. If the polyurethane foam breaks down, it can produce particles and also release chemical gases.A lot more CPAP recall legal actions are anticipated to follow because there is evidence that Philips understood concerning the issues and boosted wellness risks associated with the PE-PUR foam. In enhancement, Philip mentions they have actually been receiving grievances from users of the recalled sleep apnea makers regarding black fragments and also particles in the airpath of the clinical tools.
Philips CPAC Lawsuit
Nonetheless, they differ on where to hold the process. There are many root causes of action that can be taken against Philips, including: stringent product obligation as well as negligence You or a liked one might be permanently injured due to a faulty clinical tool manufactured by Philips. Submitting a legal action might spend for past and also future medical expenses.
A constant positive airway stress equipment, even more typically referred to as a CPAP equipment, is a clinical device that is normally recommended by physicians to deal with sleep apnea problems. There are a number of different categories of sleep apnea, consisting of obstructive sleep apnea, main rest apnea, and intricate sleep apnea syndrome. For those with rest apnea or respiratory problems, CPAP as well as Bi, PAP machines function by blowing air into people' air passages while they are asleep or seeking air.
The 20-Second Trick For Philips CPAC Lawsuit
Nevertheless, the Philips firm recalled its breathing maker due to the discovery of contaminants in the polyester-based polyurethane foam, which was picked for sound-reducing high qualities.
Why is it that Philips is now faced with class action lawsuits and private cases? The polyurethane foam was possibly never ever fit to be utilized in the maker's respiratory tract because it contains toxic chemicals.
Allegedly, Philips had gotten grievances about the foam breaking part and also being breathed in for many years. The firm did nothing to examine and boost upon the design, neither were there ever before any type of previous recalls (Philips CPAC Lawsuit). Philips has been berated by its customers for not managing the CPAP device recall correctly. Plenty of people have ceased the use of their rest apnea machines as guided and afterwards sent the influenced makers back to the manufacturer.
How Philips CPAC Lawsuit can Save You Time, Stress, and Money.
If you have actually had a rupture surgically repaired, chances are good that the doctor used mesh to aid enhance and safeguard this location. While it almost constantly functions as intended, rupture mesh can in some cases stop working, creating difficulties.

Numerous difficulties associated to rupture repair with medical mesh that have actually been reported to the FDA have been linked with recalled mesh items that are no more on here are the findings the marketplace. Pain, infection, reoccurrence, adhesion, obstruction, as well as opening are one of the most common issues connected with recalled mesh. In the FDA's analysis of medical negative occasion reports to the FDA, remembered mesh items were the major reason for digestive tract perforation as well as blockage complications.
Therefore, the only risk-free choice that Philips uses to its customersmany of whom need as well as rely on the recalled breathing machinesis to purchase Philips's newer version, profiting Philips further. The Complaint alleges that Philips has no concrete timeline for replacing the remembered CPAP machines and also various other gadgets as well as may not offer replacements for a year or more, despite the fact that patients need to use their devices every day.
The 6-Second Trick For Philips CPAC Lawsuit
A contingent charge agreement suggests this article we only earn money if we win, as well as that we will get our fees from the quantity paid by the Accused in the event. Please call us to go over the details of your situation by filling in the "Demand A Free Appointment" kind on this page.
In September 2021, Philips revealed it would fix or change recalled makers due to the problematic foam. That process might take up to a year, according to the company. Some individuals may select to ask their doctor for CPAP alternatives rather. On June 28, 2022, Philips provided a research study update regarding PE-PUR audio abatement foam screening.
Philips additionally stated equipments cleansed with ozone cleaners were 14 times more probable to have foam destruction. PE-PUR foam might cause side results because of the chemicals in the foam. Philips performed laboratory tests and also discovered a minimum of 5 harmful chemicals existing in foam bits and gases released from degraded foam.